
ستطرح شركة Vivolight Medical نظام P80-E OCT لأول مرة في مؤتمر TCT 2025 في سان فرانسيسكو
سان فرانسيسكو، أكتوبر 2025مؤتمر علاجات القلب والأوعية الدموية عبر القسطرة (TCT) هو أحد المؤتمرات العالمية الرائدة في مجال طب القلب التداخلي. ستُعقد الدورة السابعة والثلاثون من المؤتمر في مركز موسكون للمؤتمرات بسان فرانسيسكو، من 25 إلى 28 أكتوبر 2025. يجمع المؤتمر أكثر من 10,000 مشارك، منهم أكثر من 5,000 طبيب، ويضم معرضًا حافلًا يضم أكثر من 100 شركة من أكثر من 100 دولة، ما يجعله منصة عالمية رائدة يتبادل فيها الأطباء والباحثون ورواد الصناعة أحدث البيانات والابتكارات.
الظهور الأول لـ Vivolight في TCT
تُمثل فيفولايت إنجازًا هامًا في توسيع حضورها في الولايات المتحدة الأمريكية وتعزيز حضورها العالمي في مجال تصوير القلب والأوعية الدموية. وبصفتها رائدة في مجال التصوير المقطعي البصري التوافقي (OCT) في الصين، تستفيد الشركة من هذه المنصة الدولية لعرض أحدث تقنياتها في تصوير القلب والأوعية الدموية والتواصل مع الأطباء والباحثين والشركاء حول العالم.

إنجاز تنظيمي
في 11 أبريل 2025، منحت إدارة الغذاء والدواء الأمريكية موافقة 510(k) (K242098) لمنتجات Vivolight Medical كورناريس P80-Eو كورناريس موبايل-إيأنظمة التصوير المقطعي البصري، جنبًا إلى جنب مع لومين كروس F2قسطرة تصوير. تُجيز هذه الموافقة تسويق المنتجات في الولايات المتحدة الأمريكية، وتُثبت امتثالها للمعايير التنظيمية الصارمة.
لقد تم بالفعل اعتماد أنظمة التصوير OCT من Vivolight من قبل أكثر من 300 مستشفى في جميع أنحاء الصين، نالت اعترافًا سريريًا واسع النطاق لجودة صورها وموثوقيتها وسهولة استخدامها. يوفر نظاما Cornaris P80-E وCornaris Mobile-E أداءً تصويريًا عالي الدقة ومستقرًا يضاهي أداء العلامات التجارية العالمية الرائدة.
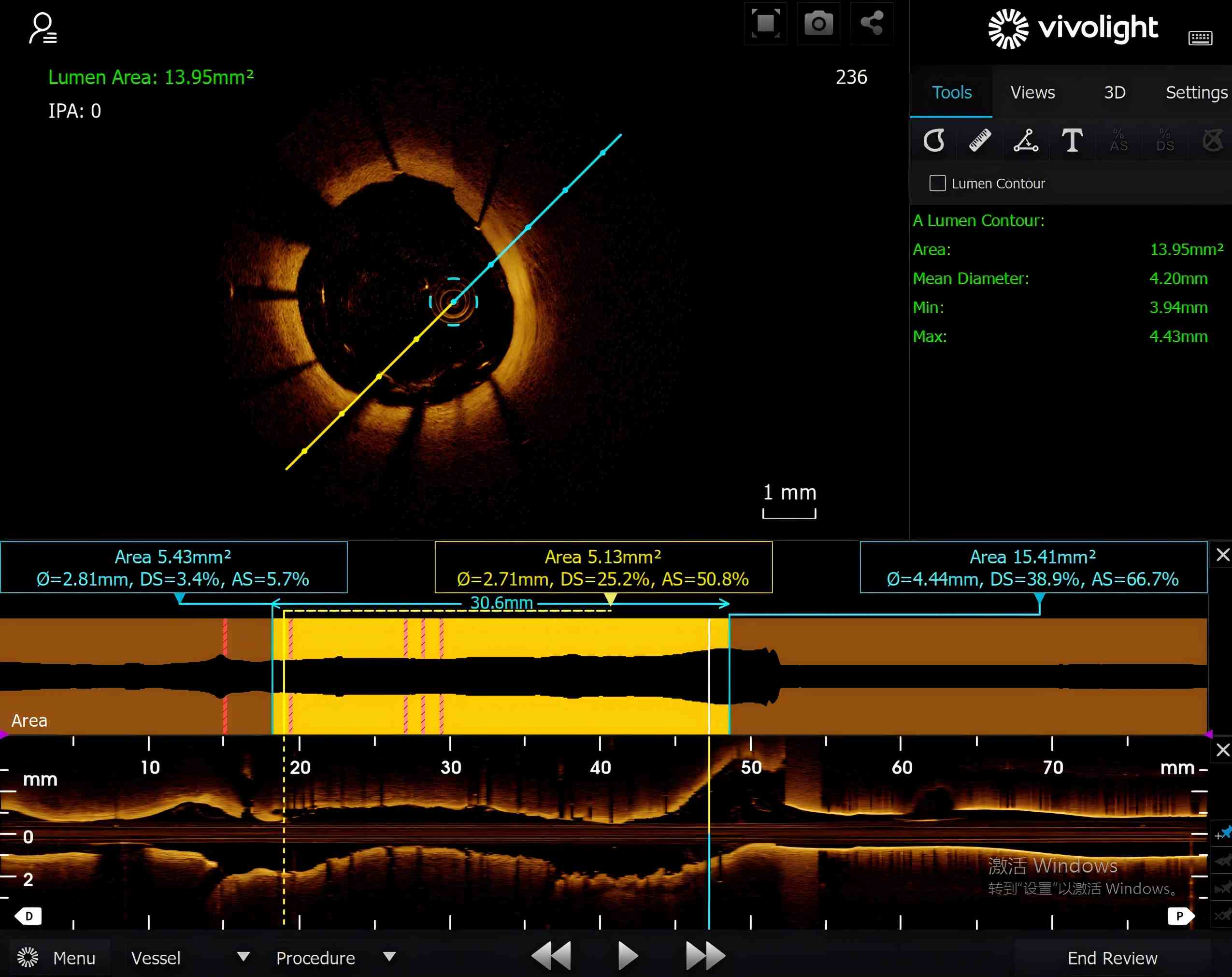
دخلت منتجات Vivolight الأسواق بما في ذلك إندونيسيا، تشيلي، أرمينيا، هونج كونج، وماكاومما يُمثل تقدمًا مطردًا في توسع الشركة عالميًا. بفضل الابتكار المستمر والتعاون السريري، تلتزم فيفولايت بتطوير تقنيات التصوير داخل الأوعية الدموية التي تُمكّن الأطباء وتُحسّن رعاية القلب والأوعية الدموية حول العالم.
خاتمة
فيفولايت ميديكال هي شركة أجهزة طبية عالية التقنية، متخصصة في تقنيات التصوير البصري للتدخلات القلبية الوعائية. من خلال أبحاثها وتطويرها المستقل، وتعاونها السريري، وابتكاراتها القائمة على الذكاء الاصطناعي، تواصل فيفولايت الارتقاء بتقنيات التصوير المقطعي البصري (OCT) إلى آفاق جديدة، مقدمةً حلولاً تعزز الدقة والكفاءة ونتائج المرضى في مجال طب القلب التداخلي.
ترك رسالة
مسح ضوئي إلى WhatsApp :
